Last week, we convened more than 300 CEOs, board members, and chief scientific and medical leaders for our third annual Wing at JPM Summit. Across multiple speakers and attendees—from pharma and providers to payors, tech, academia, government, startups, and investors—one message was consistent: healthcare is entering a forced-acceleration phase as AI becomes deployable in real workflows, cost pressure intensifies, and patient expectations rise.
Leaders were especially aligned on two near-term impacts of data and AI: an explosion of innovative medicines (new biology, modalities, and targets), and a step-change in drug design and clinical development speed—faster iteration on what works, for whom, and how to improve it. Some leaders believe this will expand precision indications, unlock previously untreatable diseases, and materially increase the number of approvals per year.
To capture how this group sees 2026 unfolding, we asked attendees to complete a predictions survey. Because the Summit is off-the-record, we’re sharing our aggregated, anonymized findings—a snapshot of what leaders believe is real, what’s stalling, and what could reshape healthcare next.
Our findings are:
- Executives predict a median 52% increase in the number of new drug approvals by the FDA in 10 years.
- AI “moonshots” cluster around primary care, personalized medicine, drug development, cancer, human biology, and workflow transformation across the healthcare ecosystem.
- Executives expect a median 15% reduction in healthcare administration costs from AI within three years, with wide dispersion in views.
- 44% of respondents believe cell and gene therapies will have the greatest impact among therapeutic modalities over the next five years—a surprising lead by a roughly 3-to-1 margin.
- Two-thirds of leaders are positive on the five-year outlook for ChatGPT Health, while roughly one-third are negative and a small minority are neutral.
- The most frequently advocated policy reforms are single payer, universal health coverage, and true data interoperability.
- Black Swan scenarios span both upside and downside, including AI breakthroughs or failures, industry consolidation or collapses, government shifts, GLP-1 shocks, China-related developments, workforce constraints, and pandemic risk.
Findings
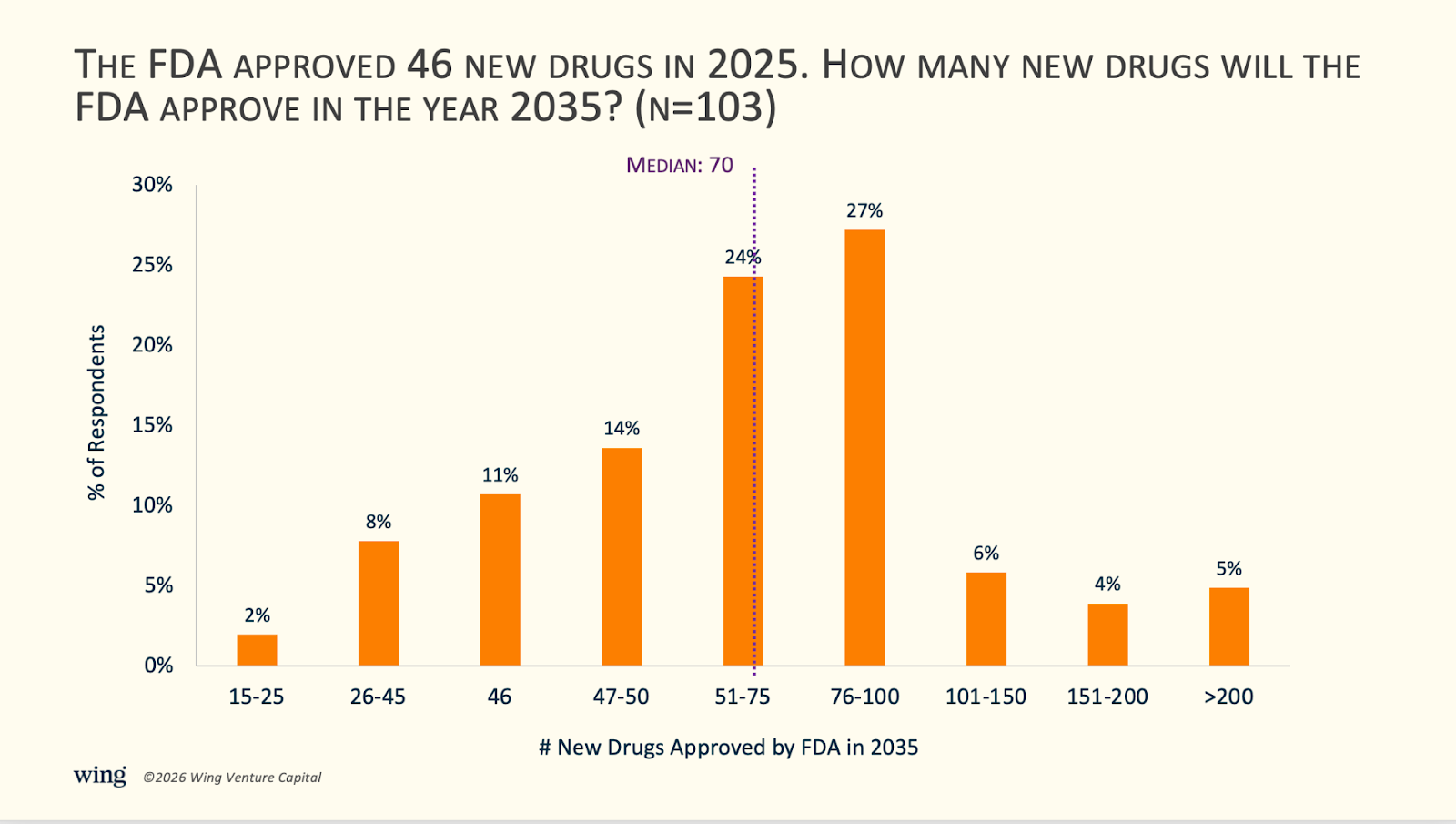
1) Executives predict a median 52% increase in the number of new drug approvals by the FDA in 10 years.
Most leaders expect the pace of approvals to rise: 79% predict an increase over the next 10 years, 11% expect approvals to stay roughly flat, and 10% anticipate a decline.
Expectations skew aggressive. 42% of respondents project approvals climbing from roughly 46 per year today to more than 76 per year. Several executives put the figure closer to roughly 100 approvals annually, driven by more precise, subgroup-specific medicines (“more frequent approvals of precision medicines for specific disease subsets”), global innovation flow-through (“China’s innovations will be reaching scale”), and a timing thesis that 2036 could mark the start of an “AI Harvest”—AI-discovered molecules finally completing the roughly 10-year clinical development cycle.
Several respondents predict a 10-100x increase in new drugs through drug platform approvals. One colleague said, “We might see an increase of classical drug approvals but more likely we will see more and more drug platform approvals that allow further personalization. We might get 60-80 classical drugs and 20-30 drug platforms approved… and the 20-30 platforms might allow the generation of 1000-5000 personalized drug derivatives.” Another executive said, “the agency will shift to increasingly approve platforms and manufacturing frameworks rather than individual patient specific treatments.”
2) AI moonshot themes include: primary care, personalized medicine, drug development, human health, human biology, cancer, and industry and company workflows.
Executives shared a wide range of AI "moonshot" ideas and applications.
On drug development, Daphne Koller, Founder and CEO of insitro, noted that the Eli Lilly–Nvidia announcement signals growing acceptance of AI for molecular design—citing molecules now advancing into Phase II and III as early proof points. "But let's be clear: these results, while meaningful, are not yet truly transformative," Koller said. "We still haven't addressed the roughly 75% of diseases with no approved therapies. That's the real benchmark, and I believe that inflection is coming."
On 2026 expectations, Greg Corrado, Distinguished Scientist at Google, said he hopes we’ll soon see researchers explicitly credit AI and LLMs as a “critical part of scientific progress,” with “seismic shifts” most likely in the drug discovery pipeline and back-office medical documentation.
.png)
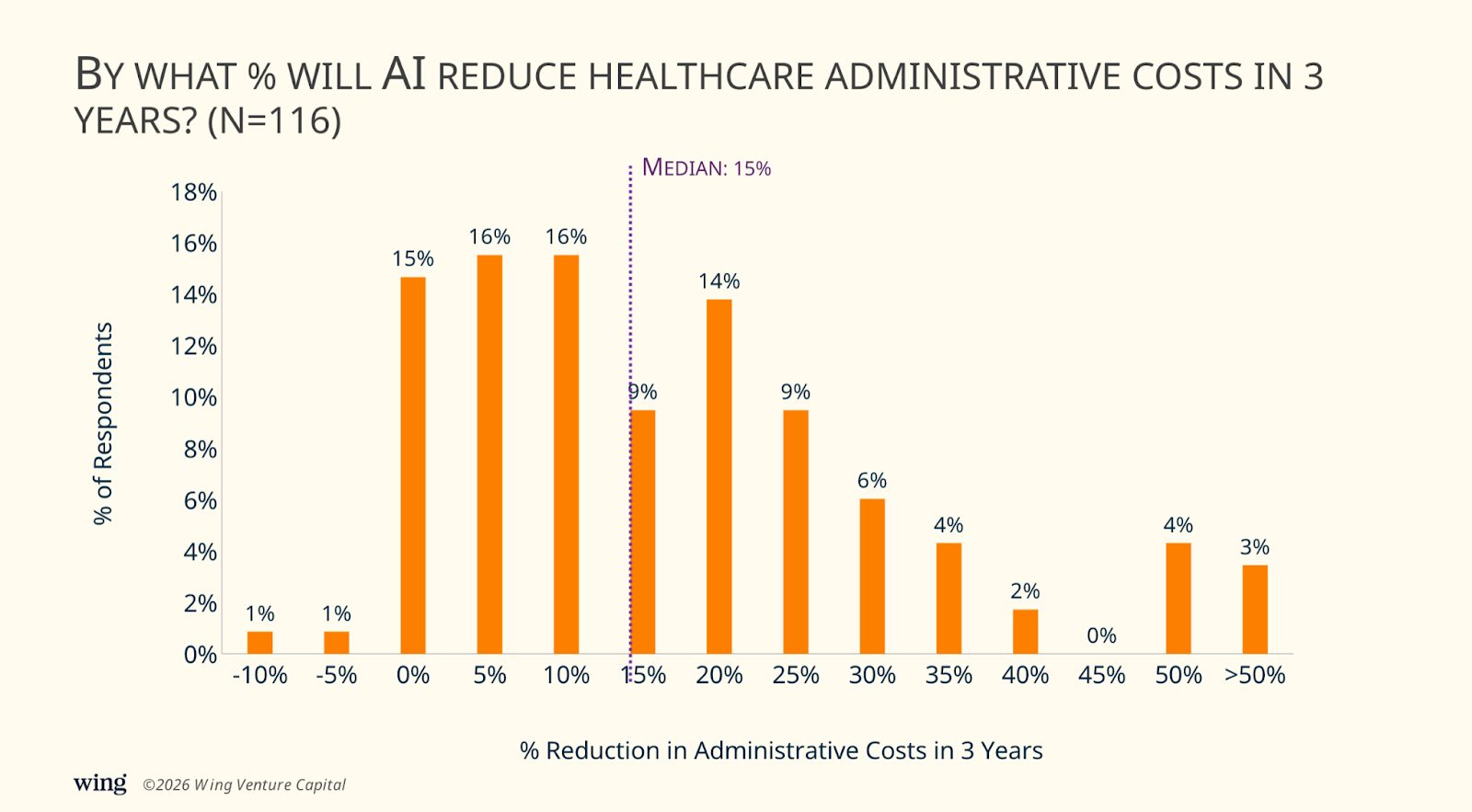
3) Executives anticipate a median 15% reduction in healthcare administration costs from AI within three years.
The distribution was wide, but the median landed at a modest 15%. 49% of leaders expect 10% or less in near-term savings, arguing that AI will relabel work more than remove it: “Admin responsibilities will change and costs will shift,” said one respondent, who predicted higher spend on “appealing insurance denials.” Others were more blunt (“0%”) citing structural inertia and policy constraints, or suggesting the best-case scenario is simply that administrative costs grow closer to GDP/inflation. As one put it: “10% because the producers of burden will not want to go quietly into the night.”
A few respondents went further and warned that AI could increase administrative costs in the short run—layering new tooling, governance, and compliance on top of existing processes.
Several leaders framed this as a timing problem: limited impact in three years, but meaningful impact over a longer horizon. One predicted “0%” in the near term due to “glitches and newsworthy privacy/safety events” slowing adoption, followed by “50%” reductions over 10 years once systems fully transition. Another echoed the same arc: “Minimal in 3 years… Massive reductions in 3+ years.”
On the more optimistic end, 38% forecast 15% to 30% reductions within three years, with some suggesting 25%–40% is plausible depending on the institution and geography (“In the U.S., by 20%. Worldwide, by 35%.”).
To us, a 15% median still feels conservative. Greg Corrado offered a sharper formulation: while dollars may lag process change, he expects “person-hours of administrative labor” to drop materially, estimating that organizations that truly adopt AI could see a 40%–50% reduction in administrative person-hours relative to patient-hours of care.
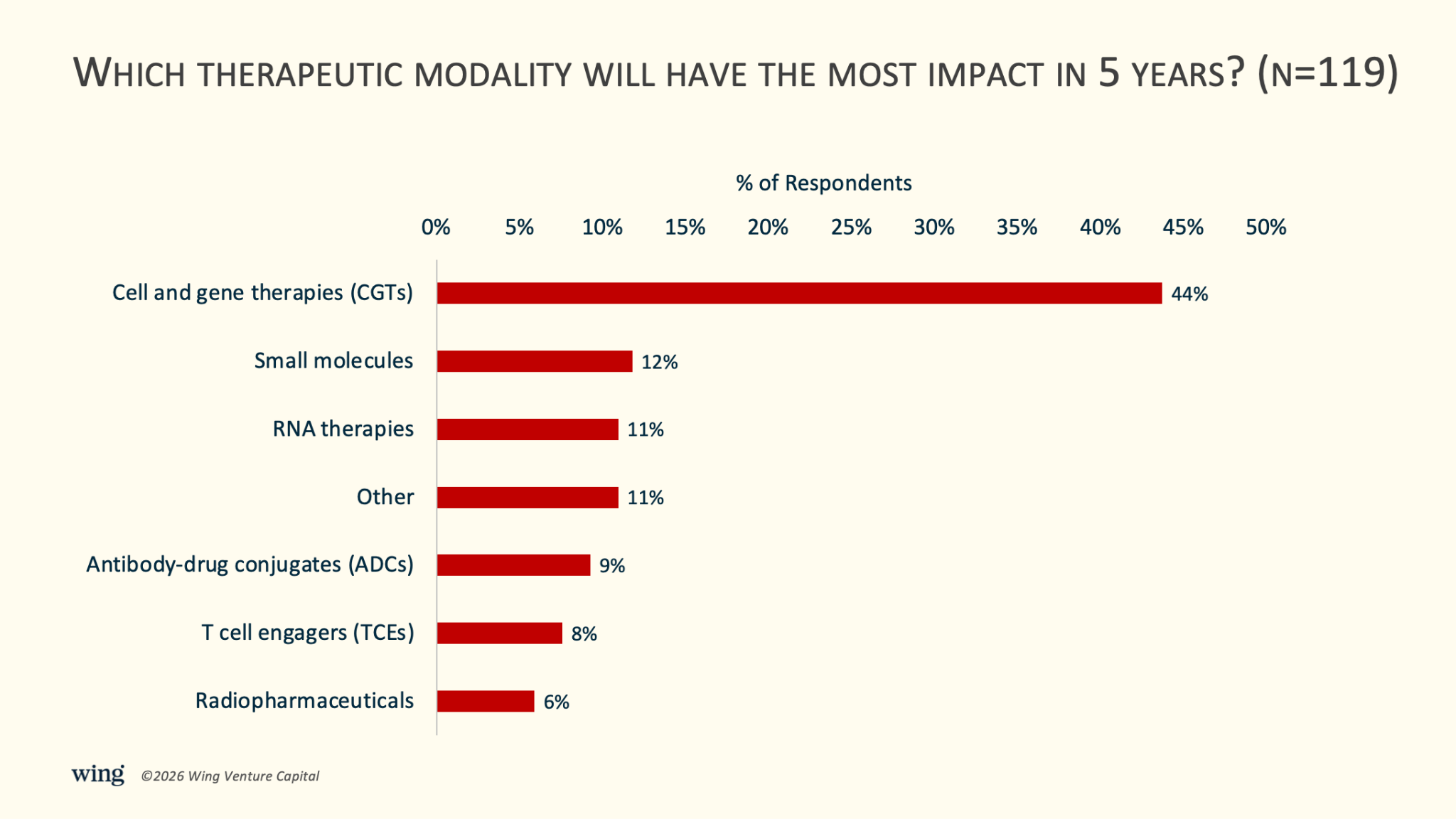
4) 44% expect CGTs to have the most impact among therapeutic modalities in five years.
This was one of the more surprising results. By a roughly 3-to-1 margin, respondents chose cell and gene therapies (CGTs) as the therapeutic modality likely to have the greatest impact over the next five years. CGTs led with 44%, followed by small molecules (12%), RNA therapies (11%), ADCs (9%), TCEs (8%), and radiopharmaceuticals (6%).
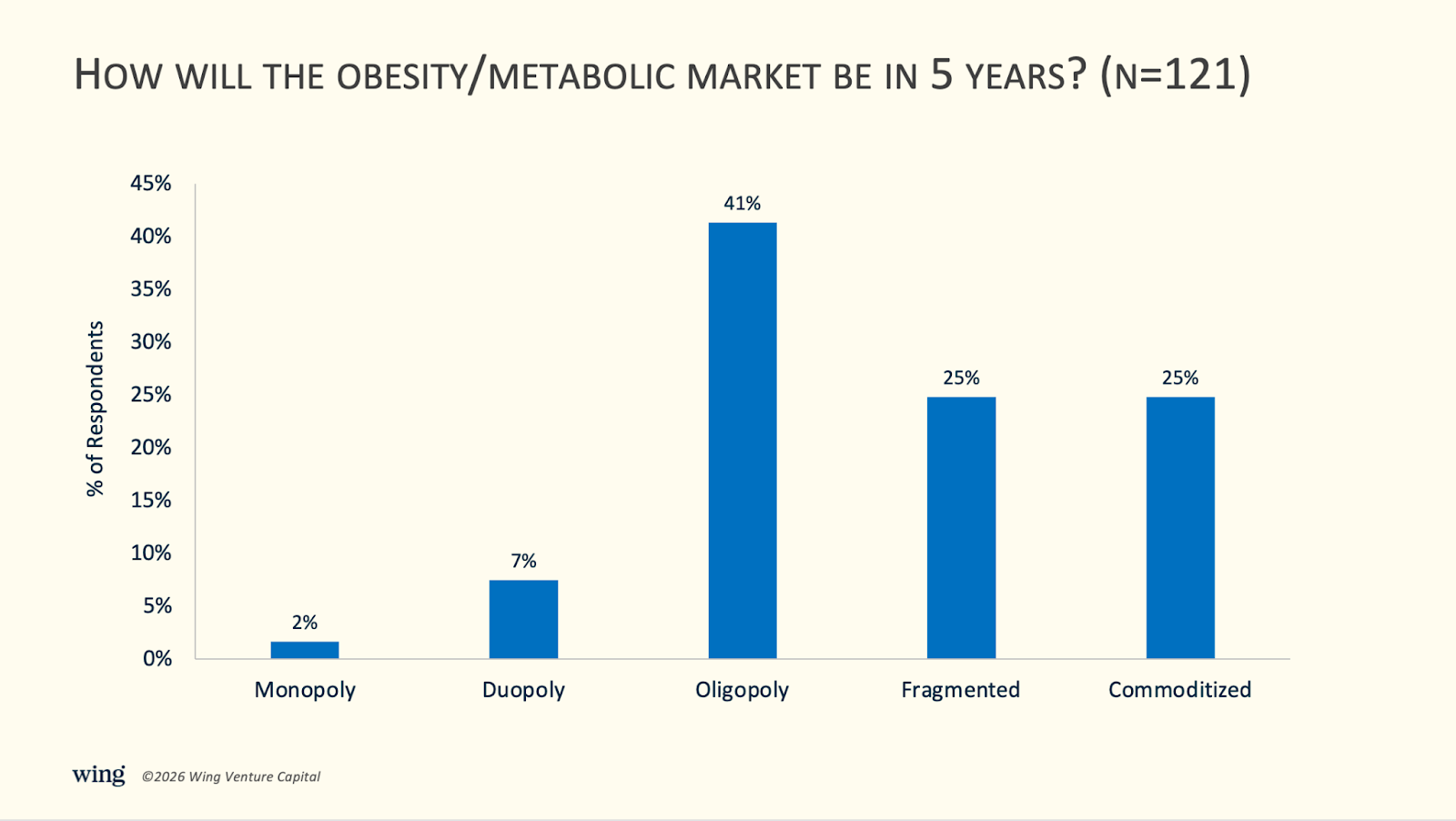
5) 50% expect the obesity/metabolic market to be fragmented or commoditized, and 41% expect it to be an oligopoly.
41% of respondents predict that the obesity/metabolic market will be an oligopoly in five years. 25% expect it to be fragmented, and 25% expect it to be commoditized. Only 9% expect the market to remain a duopoly or become a monopoly in 5 years.
6) 67% of respondents are positive on the five-year outlook for ChatGPT Health, while 31% are negative and 3% are neutral.
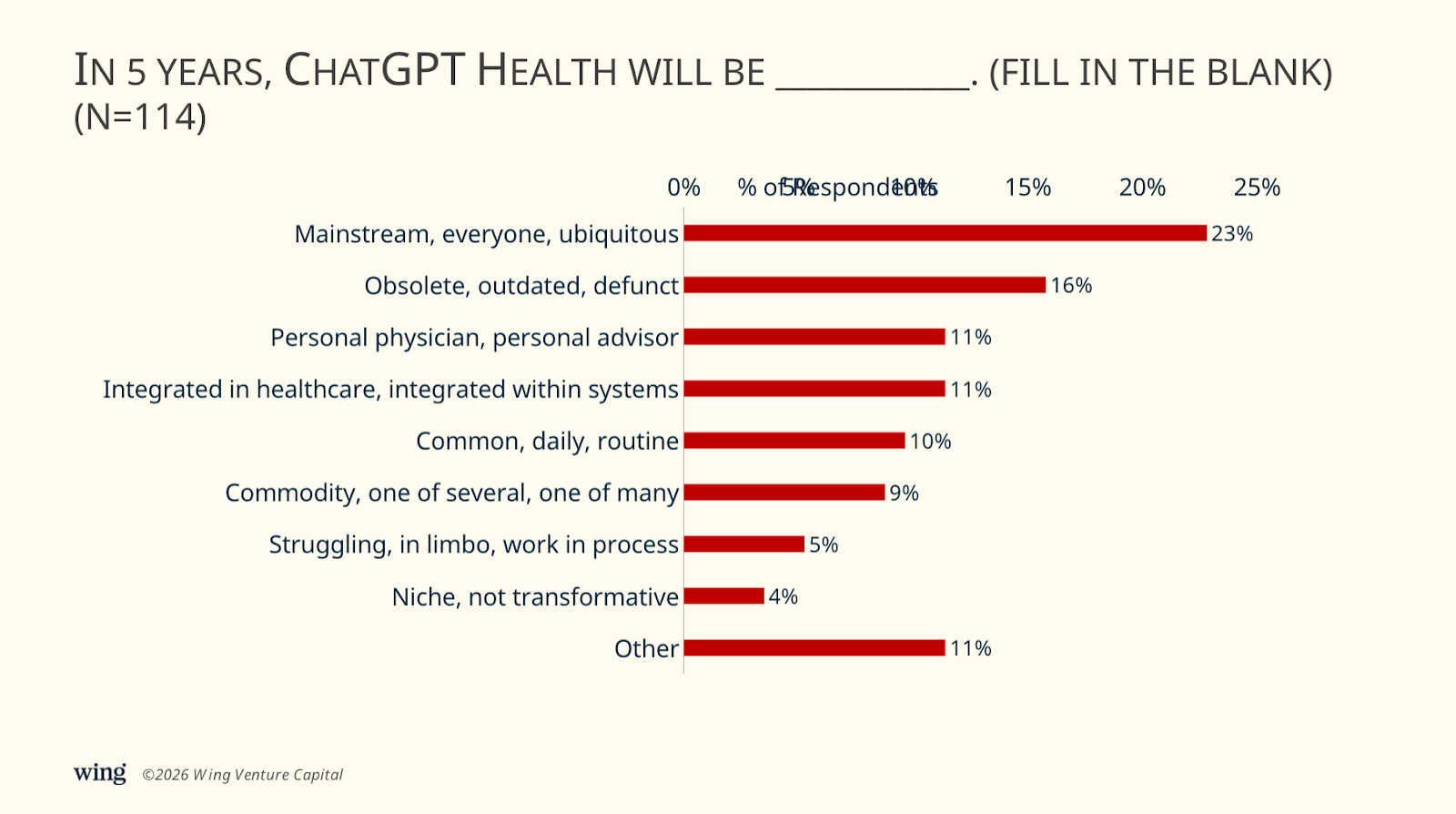
ChatGPT Health launched the week prior to the JPM Conference. 55% of executives predict it will become mainstream, integrated, or common in healthcare. “Mainstream” comments included “the go-to for everything,” “a household word,” “what Amazon has been for retail,” and “the first stop for patients and physicians to assess symptoms.” “Integrated” responses included “embedded in core healthcare platforms,” “the operating layer that coordinates clinical, administrative, and patient-facing workflows,” and “an integrated platform within 2 of the top 3 electronic health records.” “Common” predictions included “a fully integrated part of our daily work,” “my primary care physician,” “providing 85% of medical diagnoses,” and “a personal super-assistant.”
However, a meaningful minority disagree. 30% predict ChatGPT will be obsolete, commoditized, or struggling in five years. “Obsolete” perspectives included “a non-event,” “defunct,” “a forgotten product,” “overtaken by other AI modalities that are seamlessly integrated into existing workflows,” and “superseded by apps created by EMR companies.” “Commoditized” comments included “competing with at least 10 other providers offering similar or superior capabilities” and “one of many options, and not the best one, for people looking for health information.” “Struggling” thoughts included “in limbo,” “still a work in progress,” and “struggling to achieve broad EHR integration.”
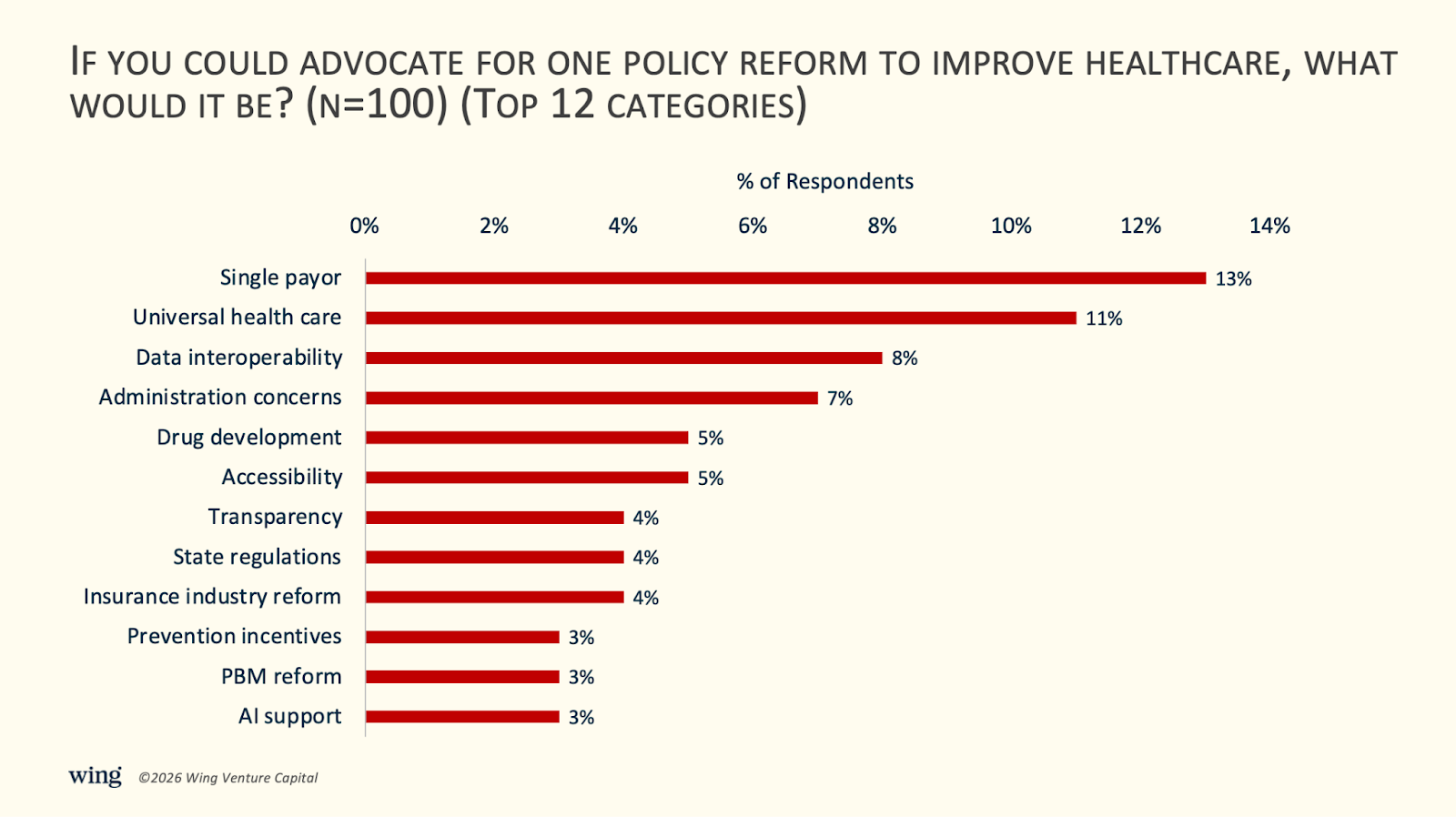
7) The top policy reforms advocated by executives are single payor, universal health care, and data interoperability.
Among surveyed leaders, the most frequently cited reforms were single payer (13%) and universal health coverage (11%)—including proposals such as “Medicare Advantage for all.” Data interoperability followed at 8%, reflecting persistent frustration with fragmented health information systems.
One executive argued for “mandatory implementation of a unified data standard… across the U.S. healthcare system,” effectively eliminating data silos between hospitals, insurers, and pharmacies—and weakening the structural lock-in created by today’s EHR ecosystem.
A smaller but notable share of respondents (5%) prioritized drug development reform, including faster clinical pathways (e.g., combining Phase I and II) with rigorous post-approval surveillance, and expanded FDA acceptance of surrogate endpoints for chronic diseases.
Other regulatory reform priorities include:
- Accessibility: “no patient copays”
- AI support: “let AI do more (like in Utah)”
- Antitrust: “break the top three payors, no ownership of tech companies and providers”
- Clinical trials: “a federal mandate that routine care costs for clinical trial participation be universally covered without prior authorization across payers”
- EHR reform: “mandate a single, all-encompassing patient medical record for every person in the U.S.”
- Payment reform: “cap commercial payment rates at 2x Medicare”
- Reimbursement reform: “require insurers to reimburse for therapies where there is efficacy data without requiring randomized trials”
- State regulations: “Eliminate state by state regulation of providers”
- Telemedicine: “mandatory reimbursement for telemedicine with no state line restrictions”
- Transparency: “improved payer policy transparency”
8) Black Swan events might include AI problems, company collapses, government shifts, GLP-1 shocks, China developments, or workforce shortage issues.
Positive Black Swan events might include AI momentum (increased role, success, acceptance, or reimbursement) or new drugs (“next blockbuster drug launch exceeding GLP1 success”).
Negative Black Swan events might include AI problems, company collapses, government shifts, GLP-1 shocks, China developments, or workforce shortage issues. In addition, 17% of respondents indicate a pandemic, epidemic, or outbreak as a Black Swan event for 2026.
When asked what will make a 100-1000x improvement in AI adoption in healthcare, Othman Laraki told us,“The ability to get paid for AI services. The single biggest problem in healthcare in the U.S. is that it’s not a liquid market where you can buy and sell services in an open market.”
.png)
Conclusion
It has never been a more exciting—and more consequential—time to be building in healthcare and biotech. What stood out at Wing at JPM was the shared orientation across the room: regulators, drug makers, clinicians, technologists, payors, and operators want to collaborate toward the same goal—better outcomes for patients.
The predictions survey reflects that optimism, especially around what data and AI can unlock: a surge of truly innovative medicines (novel biology, new modalities, better targets) and a step-change in speed across drug design and clinical development—faster iteration on what works, for whom, and how to improve it. Against that backdrop, leaders expect innovation to accelerate over the next decade (median +52% in FDA approvals), and many were surprised to see cell and gene therapies lead the modality discussion by a wide margin.
Just as important, the room’s “moonshots” reinforced a push toward individualized care—and a broader view of access that starts well before a prescription: earlier detection, chronic condition management, and proactive, preventative interventions. If stakeholders keep rowing in the same direction—aligning incentives, trust, and data rails—technology can meaningfully expand access and improve outcomes at scale.
We look forward to your thoughts and reactions. You can reach out to us at sara@wing.vc and ansu@wing.vc.
Methodology
The Wing VC report is based on our exclusive Health and Bio Leadership Network. The study was conducted between January 9, 2026 and January 11, 2026 in conjunction with Wing at JPM 2026. 39% of the 122 respondents are at pharmaceutical companies, 25% are at healthcare companies, and 21% are at technology companies.

We would like to thank Wing consultant Clayton Ramsey for his contributions to the report.

.avif)


